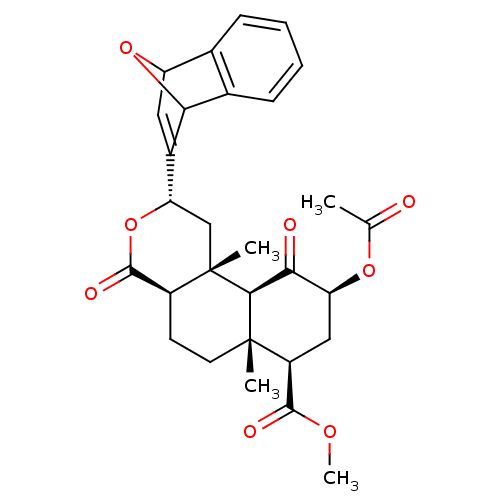
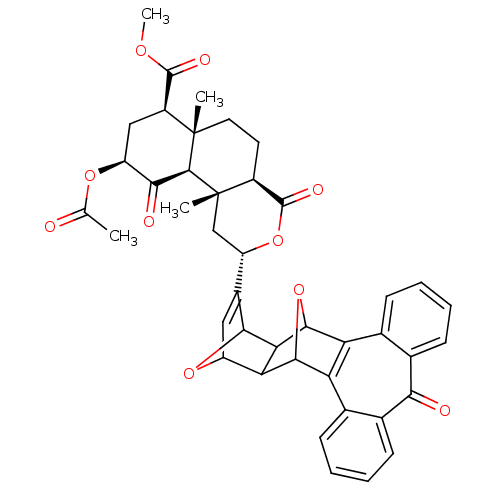
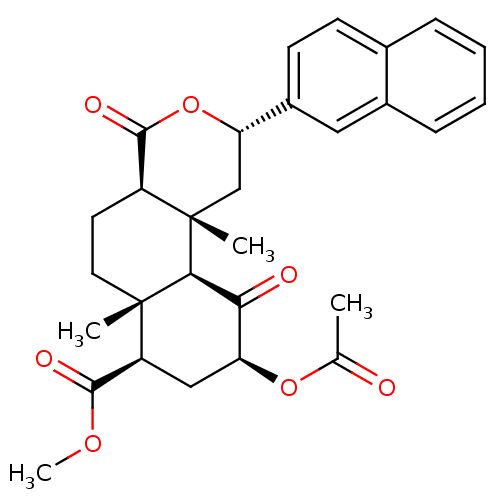
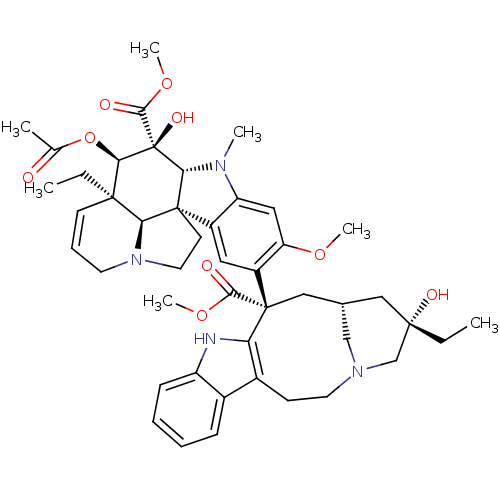
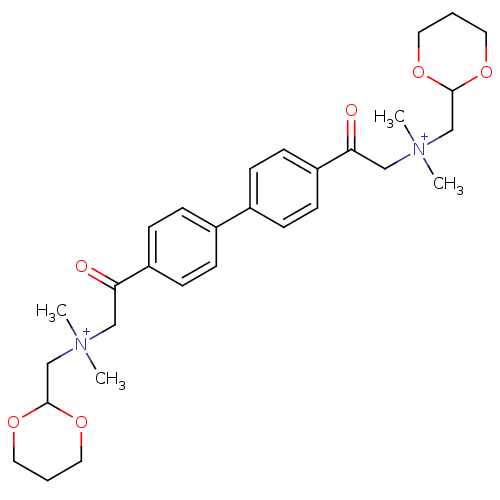
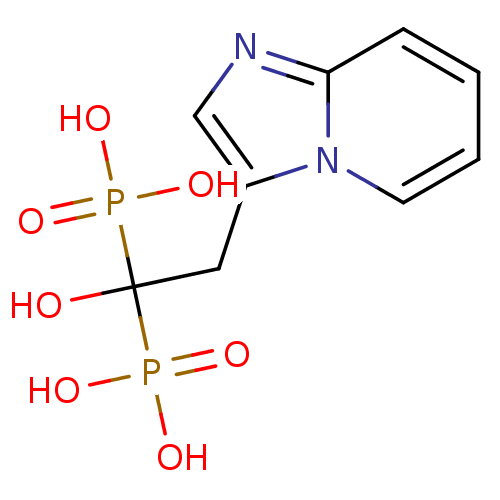
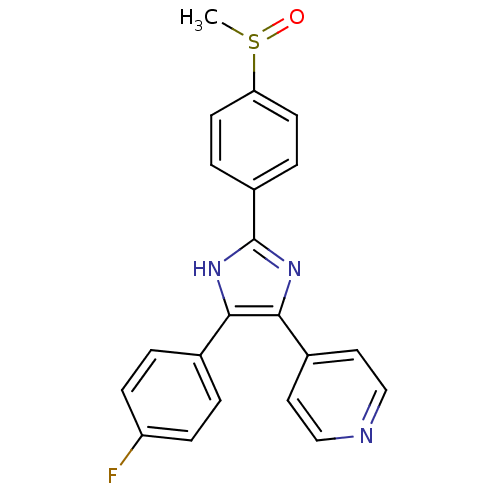
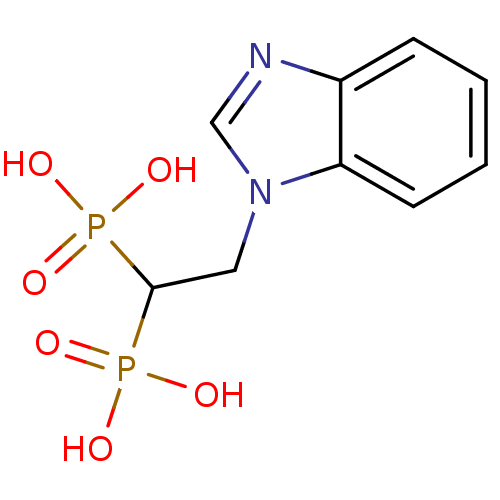
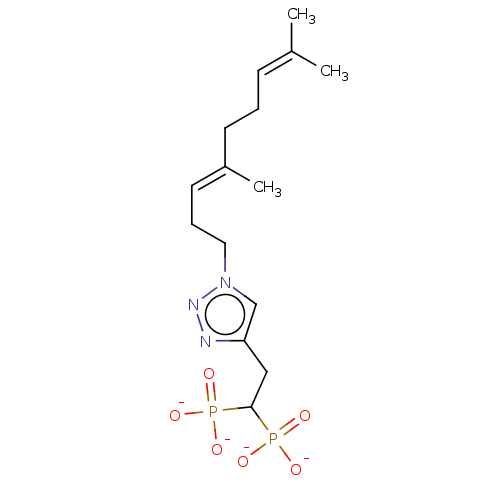
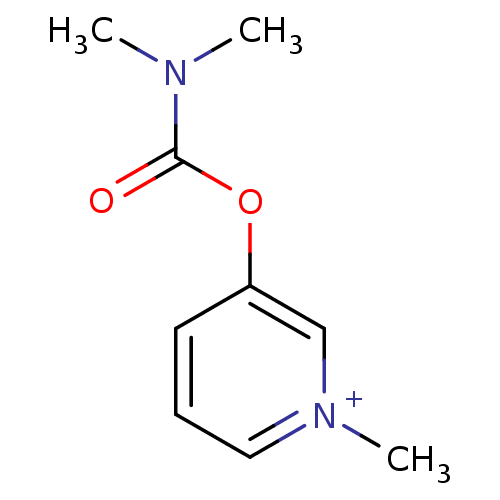
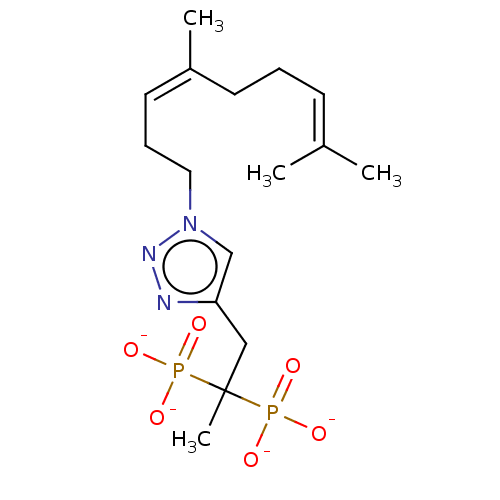
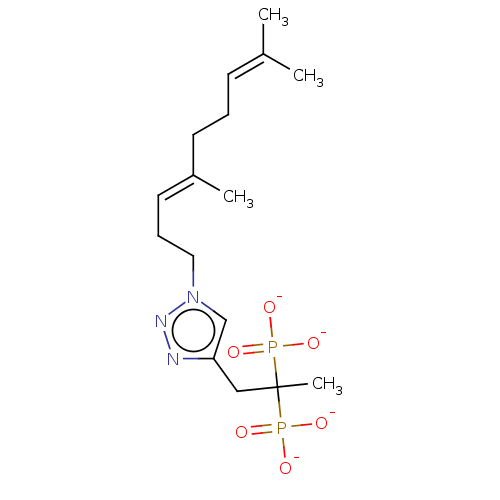
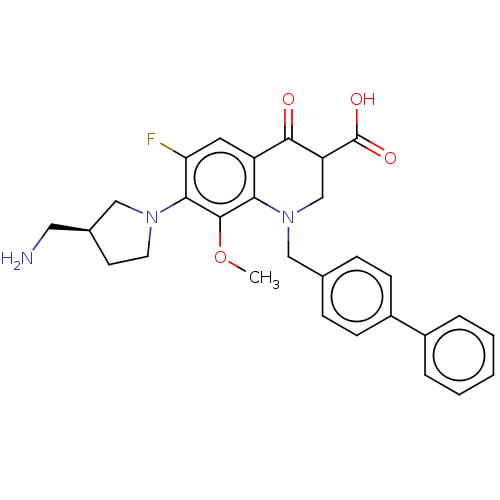
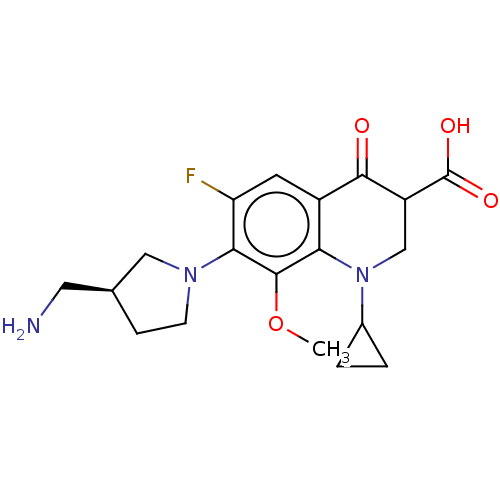
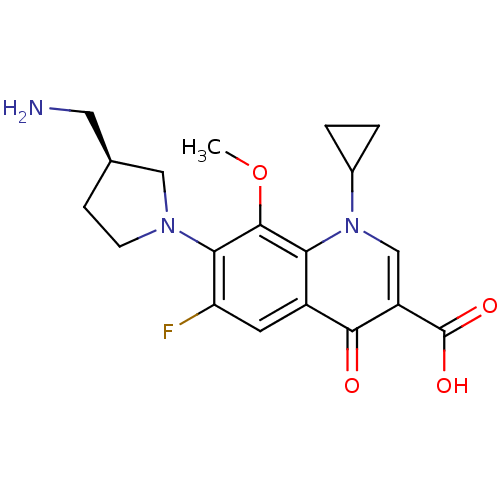
Affinity DataKi: 7.40nMAssay Description:Displacement of [3H]U69593 from kappa opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Displacement of [3H]U69593 from kappa opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 120nMAssay Description:Displacement of [3H]U69593 from kappa opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 228nMAssay Description:Displacement of [3H]U69593 from kappa opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 286nMAssay Description:Displacement of [3H]U69593 from kappa opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:Displacement of [3H]U69593 from kappa opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 790nMAssay Description:Displacement of [3H]U69593 from kappa opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.70E+3nMAssay Description:Agonist activity at mu opioid receptor assessed as stimulation of [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 2.26E+3nMAssay Description:Displacement of [3H]DADLE from delta opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >2.50E+3nMAssay Description:Agonist activity at mu opioid receptor assessed as stimulation of [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: >2.70E+3nMAssay Description:Agonist activity at mu opioid receptor assessed as stimulation of [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: >3.00E+3nMAssay Description:Agonist activity at mu opioid receptor assessed as stimulation of [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: >3.00E+3nMAssay Description:Agonist activity at mu opioid receptor assessed as stimulation of [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: >3.00E+3nMAssay Description:Agonist activity at mu opioid receptor assessed as stimulation of [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: >3.20E+3nMAssay Description:Agonist activity at mu opioid receptor assessed as stimulation of [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: >4.80E+3nMAssay Description:Agonist activity at mu opioid receptor assessed as stimulation of [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Displacement of [3H]DADLE from delta opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Displacement of [3H]DADLE from delta opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Displacement of [3H]DADLE from delta opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Displacement of [3H]DADLE from delta opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Displacement of [3H]DADLE from delta opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >5.20E+3nMAssay Description:Displacement of [3H]DADLE from delta opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >8.00E+3nMAssay Description:Displacement of [3H]U69593 from kappa opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]DADLE from delta opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]DADLE from delta opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.30E+4nMAssay Description:Displacement of [3H]U69593 from kappa opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 7.70E+4nMAssay Description:Inhibitory activity of the compound against Monoamine oxidase B isolated from beef liver mitochondria was determinedMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Concentration required to inhibit hydrolytic activity of bovine erythrocyte acetylcholinesterase by 50%More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of FDPS (unknown origin)More data for this Ligand-Target Pair
In DepthDetails
Article
BindingDB Entry DOI: 10.7270/Q2JM2DW5PubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)
BindingDB Entry DOI: 10.7270/Q2JM2DW5PubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)Affinity DataIC50: 3nMAssay Description:Inhibition of FDPS (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of FDPS (unknown origin)More data for this Ligand-Target Pair
In DepthDetails
Article
BindingDB Entry DOI: 10.7270/Q2M61MZNPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)
BindingDB Entry DOI: 10.7270/Q2M61MZNPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)Affinity DataIC50: 10nMAssay Description:Inhibition of FDPS (unknown origin)More data for this Ligand-Target Pair
In DepthDetails
Article
BindingDB Entry DOI: 10.7270/Q2JM2DW5PubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)
BindingDB Entry DOI: 10.7270/Q2JM2DW5PubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)TargetReceptor-interacting serine/threonine-protein kinase 2(Homo sapiens (Human))
University of Iowa
Curated by ChEMBL
University of Iowa
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of human receptor-interacting serine-threonine kinase 2 (RIPK2) at 100 uM ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibition of FDPS (unknown origin) using [14C] IPP and GPP as substrate preincubated for 10 mins followed by substrate addition and measured after 3...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Concentration required to inhibit hydrolytic activity of Acetylcholinesterase by 50%More data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Inhibition of Mitogen-activated protein kinase 14 (P38 alpha) at 100 uM ATPMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
University of Iowa
Curated by ChEMBL
University of Iowa
Curated by ChEMBL
Affinity DataIC50: 45nMAssay Description:Inhibition of recombinant GGDPS (unknown origin) assessed as radiolabeled GGPP formation preincubated for 10 mins followed by addition of 10 uM FPP s...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Concentration required to inhibit hydrolytic activity of bovine erythrocyte acetylcholinesterase by 50%More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
University of Iowa
Curated by ChEMBL
University of Iowa
Curated by ChEMBL
Affinity DataIC50: 75nMAssay Description:Inhibition of recombinant GGDPS (unknown origin) using FPP as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins ...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
University of Iowa
Curated by ChEMBL
University of Iowa
Curated by ChEMBL
Affinity DataIC50: 75nMAssay Description:Inhibition of recombinant GGDPS (unknown origin) using [14C] IPP and FPP as substrate preincubated for 10 mins followed by substrate addition and mea...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
University of Iowa
Curated by ChEMBL
University of Iowa
Curated by ChEMBL
Affinity DataIC50: 86nMAssay Description:Inhibition of recombinant GGDPS (unknown origin) using FPP as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins ...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
University of Iowa
Curated by ChEMBL
University of Iowa
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of GST-tagged human recombinant GGDPS expressed in BL21 gold bacteriaMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
University of Iowa
Curated by ChEMBL
University of Iowa
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of recombinant GGDPS (unknown origin) using FPP as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins ...More data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
University of Iowa
Curated by ChEMBL
University of Iowa
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of human recombinant GGDPS expressed in BL21 gold bacteriaMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
University of Iowa
Curated by ChEMBL
University of Iowa
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of recombinant GGDPS (unknown origin) using FPP as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins ...More data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Inhibition of Escherichia coli DNA gyrase subunit GyrA/GyrB supercoiling activity using relaxed pBR322 DNA incubated for 15 mins by ethidium bromide ...More data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Inhibition of Escherichia coli DNA gyrase subunit GyrA/GyrB supercoiling activity using relaxed pBR322 DNA incubated for 15 mins by ethidium bromide ...More data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Inhibition of Escherichia coli DNA gyrase assessed as supercoiling activity by fluorescence assay relative to controlMore data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Inhibition of Escherichia coli DNA gyrase subunit GyrA/GyrB supercoiling activity using relaxed pBR322 DNA incubated for 15 mins by ethidium bromide ...More data for this Ligand-Target Pair
Affinity DataIC50: 124nMAssay Description:Inhibition of human casein kinase 1 delta at 100 uM ATPMore data for this Ligand-Target Pair